Overview
Features
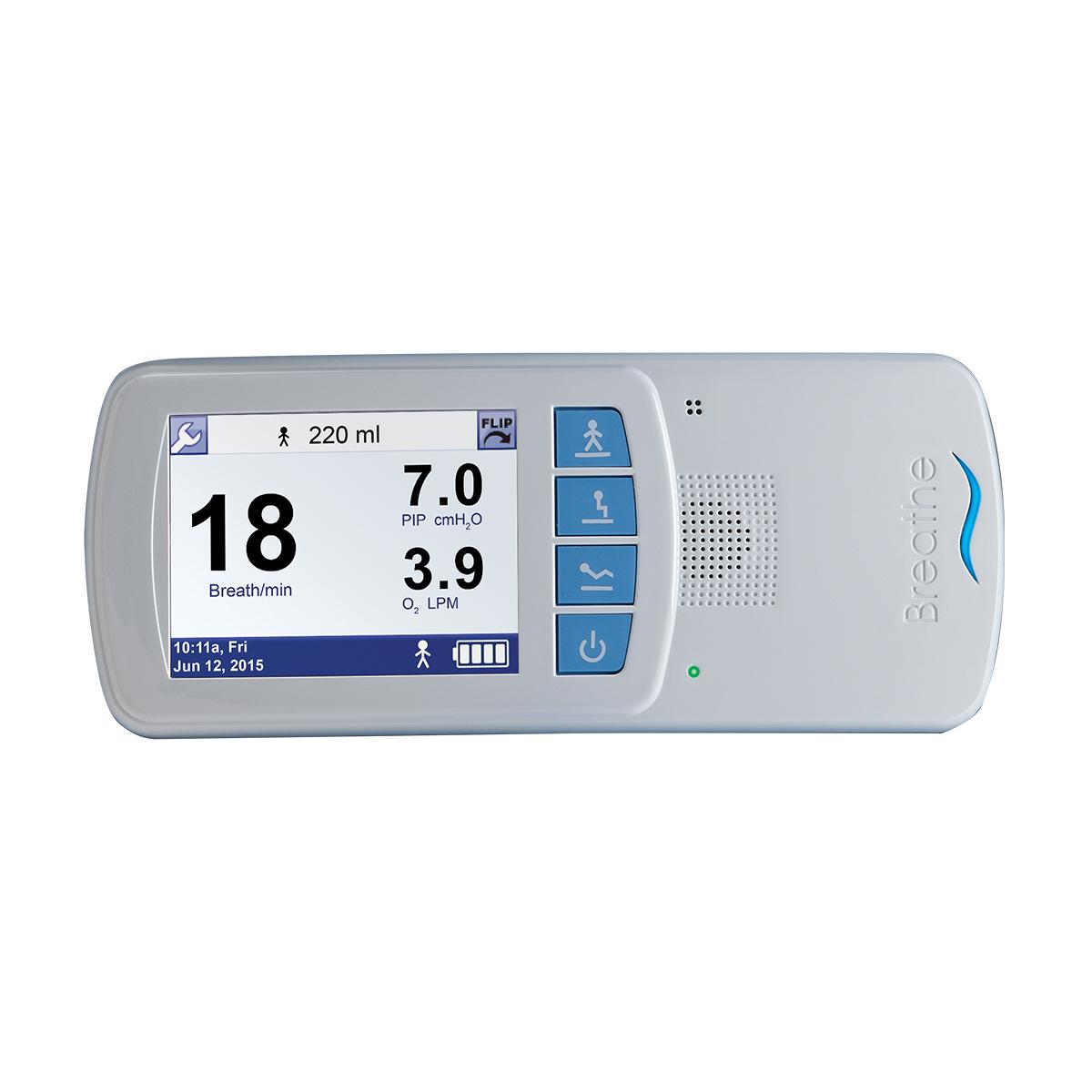
Wearable
This home ventilator device features a belt clip and a carry case.
Modular
The Life2000 System can be conveniently used at home or on the go.
Mask-Free
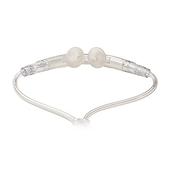
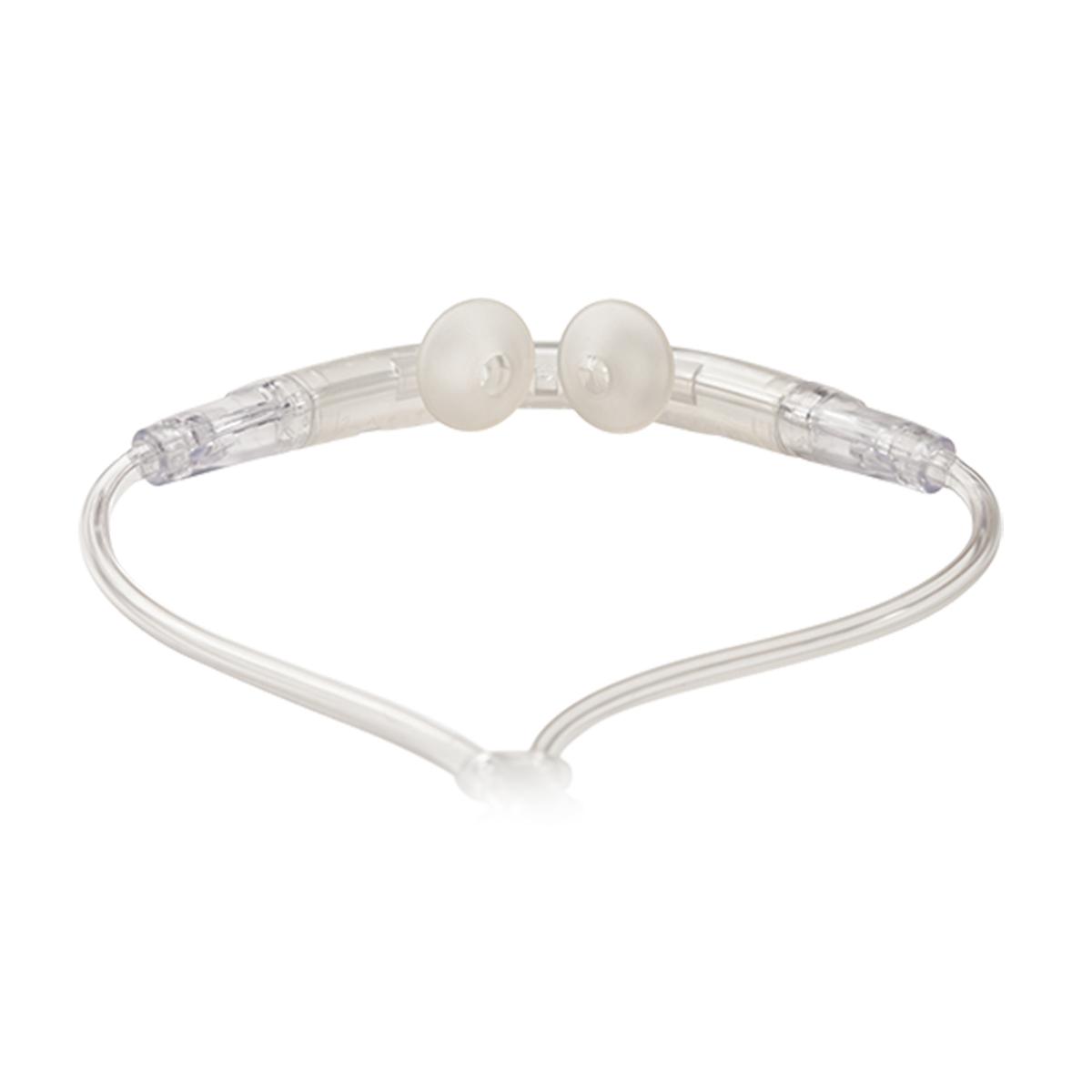
The Breathe Pillows Interface provides non-invasive ventilation.
Who can benefit?

The Life2000 Ventilation System is for patients who:
Have chronic respiratory failure due to COPD
Suffer from neuromuscular diseases or restricted thoracic disorders (e.g., kyphoscoliosis)
Are preparing for or recovering from a lung transplant
Have experienced multiple exacerbations
Have reduced oxygen saturation with minimal exercise
Comfortable Breathe Pillows Interface

The Life2000 Ventilation System is available with comfortable and discreet Breathe Pillows Interface.
Small-diameter tubing (5mm vs. traditional 22mm)
Patients can talk while wearing the noninvasive Breathe Pillows Interface
Comfortable fit for around-the-clock use
Available in multiple sizes
Breathe Universal Circuit Connector

The Life200 Ventilation System features a circuit that is compatible with any third-party interface (including full face mask, tracheostomy tube, and intubation tube).
Ability to switch masks throughout the day and night
Easier transition to home from hospital/acute settings
Versatile configurations and adjustable settings for continuous ventilation

The Life2000 Ventilation System provides three configurations for optimal versatility:
Stationary
Wearable inside the home with extended range - small-diameter, up-to-50-foot tubing allows for easy mobility
Wearable outside the home as stand-alone ventilation with an alternate pressure source
Patient-selectable prescription settings

Three patient-selectable prescription settings for rest, moderate, or high activity
Allows for continuous, around-the-clock use
Adaptable to patients’ ventilatory demands and breathing patterns
Requires less frequent prescription adjustments by clinicians
Reimbursement and support
The Life2000 Ventilation System is covered by Medicare and commercial insurance plans for invasive and non-invasive ventilators.
HCPCS Codes: E0465 and E0466
Our team of respiratory therapists work directly with physicians and other healthcare providers to ensure the Life2000 Ventilation System supports optimal clinical outcomes.
How has the Life2000 ventilator changed lives?
Technical Specifications
Dig deeper into product attributes to see how we can fit your requirements.
| Ventilation Modes | Assist, Assist/Control, Control with Apean backup |
| Breathing Type | Mandatory or Assisted |
| Tidal Volume | Up to 2000 mL (via Venturi effect) |
| Maximum Positive Inspiratory Pressure (PIP) | 40 cmH2O |
Education & Documentation
Get in the know to get the most value out of your solution.
Learn how the Life2000 Ventilation System supports breathing.
View this intro to the Life2000 Ventilation System's capabilities and configurations.
How to use: Introduction
How to use: Extended Range Configuration
How to use: Stand-Alone Configuration
How to use: Cleaning & Maintenance
How to use: Titration
How to use: Alarms & Troubleshooting
Product Documentation
-
Coverage Criteria
keyboard_arrow_downLife2000 Coverage Criteria Flyer
-
Who are ideal patients for the Life2000 Ventilation System?
keyboard_arrow_downIdeal patients are those who have been diagnosed with chronic respiratory failure (CRF) consequent to COPD, whose symptoms have progressed to the point that they experience shortness of breath with even minimal exercise, whose activities of daily living have been curtailed by their dyspnea, whose test results have indicated reduced pulmonary function (FEV1 < 50%), and/or who have a history of CRF/COPD exacerbations. Patients who want to maintain their freedom of activity are typically those who experience the best results.
-
Which patients are not ideal candidates for the Life2000 Ventilation System?
keyboard_arrow_downPatients who do not need oxygen therapy, or who tolerate point-of-care (POC) therapy with and without exertion are not ideal candidates. Additionally, patients who have physical limitations, or who are not motivated to stay active may not be the best candidates for the Life2000 Ventilation System.
-
Can the Life2000 Ventilation System be used away from home?
keyboard_arrow_downYes. The device may be connected to an oxygen cylinder with a 50 psi regulator for respiratory support outside the home.
-
How long is the Life2000 Ventilation System's battery life?
keyboard_arrow_downIn normal use, the battery's charge lasts approximately 5 to 6 hours.
-
What interface is available with the Life2000 Ventilation System?
keyboard_arrow_downThe unit uses our mask-free Breathe® Pillows Interface. It's available in four sizes—XS, S, M, and L—and is fitted to the patient during set-up.
-
What is Proportional Open Ventilation® (POV® ) technology?
keyboard_arrow_downPOV® technology refers to the method used to ventilate the patient. The interface's Venturi system entrains room air which, combined with the device's set volume and your patient's own effort, yields its total delivered tidal volume.
-
Can the Life2000 Ventilation System be used during the day and at night?
keyboard_arrow_downThe device can be used day or night, or both, and the prescribing clinician may indicate how they wish the patient to use the device.
Frequently Asked Questions
Expand All
Evidence & Outcomes
Dr. Silverman shares Life2000 Ventilation System’s positive impact on his patients’ quality of life
Hear about Dr. Brown’s experience with wearable Open Ventilation
Reduces dyspnea and increases exercise endurance for patients with COPD
Hillrom’s wearable and open ventilation* decreases work of breathing (WOB), respiratory muscle activation, and dyspnea, allowing for improved exercise tolerance and 6-minute walk tests (6MWT) compared to traditional oxygen therapy.1-6

Reduction in healthcare costs and healthcare utilization such as inpatient admissions and mechanical ventilation days7

Reduction in patient-reported CAT and mMRC scores2

Reduction in WOB1
46% reduction in accessory respiratory muscle activation3
28% reduction in Borg Dyspnea Scale3
54% increase in exercise endurance from 11.4 to 17.5 minutes (p<.001)3
34 to 73 m improvement in 6MWT distance4,5
85% improvement in ability to perform activities of daily living6
Impact of improving mobility and activity levels
Activity for 2 hours a week reduces hospital admissions and respiratory mortality by 30% to 40%, according to a 20-year follow-up study of 2,386 subjects with COPD8
Outdoor activity increases 4-year survival 35% versus 15% for oxygen-dependent patients9
Physical activity is the best predictor of all-cause mortality in COPD patients; for every 0.14 decrease in physical activity level (PAL), the relative risk of death is more than doubled10
*The data presented are reflective of studies performed on open ventilation technology.
References
1. Siobal M, Marelowe J. Work of breathing using non-invasive open ventilation in a low compliance high minute ventilation lung model. [abstract]. AARC 2015.
2. Carlin BW, Casey L, Farberow K. Improvement in health status of patients with respiratory insufficiency with the use of a noninvasive open ventilation system. Chest. 2014;146(4 MeetingAbstracts):341A.
3. Porszasz J, Cao R, Morishige R, et al. Physiologic effects of an ambulatory ventilation system in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;188(3):334–342.
4. Hilling L, Cayou C, Wondka T, Kops R. Improved 6MWT distance with a highly portable non-invasive ventilator [abstract]. Am J Respir Crit Care Med. 2010;181:A1198.
5. Garvey C, Hilling L, Cayou C, Escobar R, Heron G, McCabe L. Open, noninvasive ventilation using a 1 lb ventilator, oxygen, and a low profile mask improves 6MWT distances in advanced COPD [abstract]. Am J Respir Crit Care Med. 2011;183:A3971.
6. Carlin BW, Wiles KS, McCoy RW, et al. Effects of a highly portable noninvasive open ventilation system on activities of daily living in patients with COPD. J COPD F. 2015;2(1):35-47.
7. Morishige R, Farberow K, MacIntyre N. Health care utilization and respiratory health status in patients with chronic respiratory insufficiency following addition of a portable noninvasive ventilator to the treatment regimen. Chest. 2015;148(4 MeetingAbstracts):908A.
8. Garcia-Aymerich J, Lange P, Benet M, et al. Regular physical activity reduces hospital admission and mortality in chronic obstructive pulmonary disease: a population-based cohort study. Thorax. 2006;61:772-778.
9. Ringbaek TJ, Lange P. Outdoor activity and performance status as predictors of survival in hypoxemic chronic obstructive pulmonary disease (COPD). Clin Rehabil. 2005;19(3):331-338.
10. Waschki B, Kirsten A, Holz O, et al. Physical activity is the strongest predictor of all-cause mortality in patients with COPD: a prospective cohort study. Chest. 2011;140(2):331-342.